Raoult's Law For Non Volatile Solute - Raoult S Law For Non Volatile Solute Youtube
Calculate the vapor pressure of a solution made by dissolving 60 g of sodium chloride (nacl) in 400 g of water (h 2 o). By the way, at this introductory level, we will only discuss solutions with two volatile components. The decrease in vapor pressure is directly proportional to the mole fraction of solute in an ideal solution; ← prev questionnext question → This implies both the solute and the solvent takes the same amount of energy to escape to the vapour phase as when they are in their pure states. The vapour pressure of any component at given temperature is the product of mole fraction of the component in solution with vapour pressure of the component in pure state. Describes raoult's law and gives specific examples. I know that raoult's law holds true only for a non volatile solute in a volatile solvent mixture wherein the vapour pressure of the solution gets lowered due to the addition of solute. 1 mole h 2o weighs 18 g. Raoults law for volatile solutes. For a binary mixture of two volatile component a and b with mole fraction xa and xb with pure component vapour pressure respectively.
The decrease in vapor pressure is directly proportional to the mole fraction of solute in an ideal solution; 100% of the nonvolatile solute stays in solution, none of it enters the vapor above the solution. Unable to watch the video, please try another server. In a solution with a nonvolatile solute, only the pure vapor of the solvent is present above the solution. Raoult's law for non volatile solute

100% of the nonvolatile solute stays in solution, none of it enters the vapor above the solution.
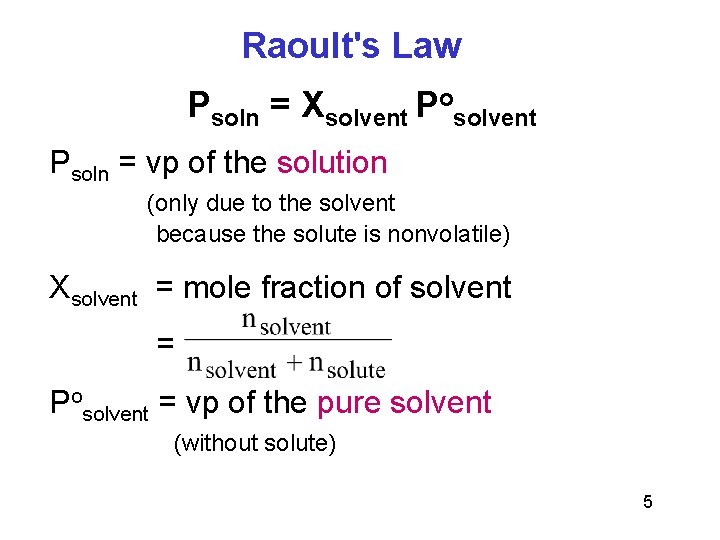
Raoult's law states that the relative lowering of vapour pressure of a solution containing a nonvolatile solute is equal to the mole fraction of the solute in the solution. I know that raoult's law holds true only for a non volatile solute in a volatile solvent mixture wherein the vapour pressure of the solution gets lowered due to the addition of solute. Therefore, the vapor pressure of the solution will be the vapor pressure due to solvent only. Now, first of all does it hold for a mixture of immiscible liquids ? P solution = (p solv o) (χ solv) Raoults law for volatile solutes. B) (i) 1 mole c 12h 22o 11 weighs 342 g; According to raoult's law, for dilute solutions containing non volatile solute, the relative lowering of vapor pressure, δp/p o is equal to the mole fraction of solute, x 2. Unable to watch the video, please try another server. (1) p s o l u t i o n = χ s o l v e n t p s o l v e n t o In a solution with a nonvolatile solute, only the pure vapor of the solvent is present above the solution. This implies both the solute and the solvent takes the same amount of energy to escape to the vapour phase as when they are in their pure states.
Therefore, the vapor pressure of the solution will be the vapor pressure due to solvent only. Raoult's law and non volatile solutes; Vapour pressure of solutions of liquids in liquids
(1) p s o l u t i o n = χ s o l v e n t p s o l v e n t o
Describes raoult's law and gives specific examples. In a solution with a nonvolatile solute, only the pure vapor of the solvent is present above the solution. (1) p s o l u t i o n = χ s o l v e n t p s o l v e n t o When the intermolecular forces of attraction between dissimilar molecules. Raoult's law is a thermodynamic law that states that the partial vapor pressure of each component of an ideal mixture of liquids equals the vapor pressure of the pure component multiplied by the. Now, first of all does it hold for a mixture of immiscible liquids ? Also give any two limitations of raoult's law. Raoult's law is valid only in the case of ideal solutions. P solution = (p solv o) (χ solv) Calculate the vapor pressure of a solution made by dissolving 60 g of sodium chloride (nacl) in 400 g of water (h 2 o). For a binary mixture of two volatile component a and b with mole fraction xa and xb with pure component vapour pressure respectively. Raoult's law states that the vapor pressure of a solvent above a solution is equal to the vapor pressure of the pure solvent at the same temperature scaled by the mole fraction of the solvent present: B) (i) 1 mole c 12h 22o 11 weighs 342 g; The decrease in vapor pressure is directly proportional to the mole fraction of solute in an ideal solution;
All of the unknown substance remains in solution. Raoult's law and non volatile solutes; Describes raoult's law and gives specific examples. Phases of matter study cards; When the intermolecular forces of attraction between dissimilar molecules. For a binary mixture of two volatile component a and b with mole fraction xa and xb with pure component vapour pressure respectively.
(b) let a volatile liquid b be added having mole fraction xb then according to raoult's law, pb = pb0 xb total pressure (p) = pa + pb = pa0 xa + pb0 xb
Please log inor registerto add a comment. 1 mole h 2o weighs 18 g. Raoult's law there are several ways of stating raoult's law, and you tend to use slightly different versions depending on the situation you are talking about. By the way, at this introductory level, we will only discuss solutions with two volatile components. The raoult's law states that, the vapour pressure of solvent over the solution is equal to the vapour pressure of pure solvent multiplied by its mole fraction in the solution. concept: For a binary mixture of two volatile component a and b with mole fraction xa and xb with pure component vapour pressure respectively. P solution = (p solv o) (χ solv) Phases of matter study cards; Raoults law for volatile solutes. Therefore, the vapor pressure of the solution will be the vapor pressure due to solvent only. The lowering of vapor pressure depends on the mole fraction of the solute dissolved and the vapor pressure of the pure solvent. Raoult's law states that the vapor pressure of a solvent above a solution is equal to the vapor pressure of the pure solvent at the same temperature scaled by the mole fraction of the solvent present:
The decrease in vapor pressure is directly proportional to the mole fraction of solute in an ideal solution; raoult's law. Unable to watch the video, please try another server.

For a nonvolatile solute bin a liquid solvent a, the relationship between concentration and vapor pressure is expressed by raoult's law:

Raoult's law is valid only in the case of ideal solutions.

(b) let a volatile liquid b be added having mole fraction xb then according to raoult's law, pb = pb0 xb total pressure (p) = pa + pb = pa0 xa + pb0 xb

Raoults law for volatile solutes.

P solution = (p solv o) (χ solv)

Describes raoult's law and gives specific examples.

Nonvolatile and volatile solutes 16 of 20 > review constants periodic table as the concentration of a solution increases its vapor pressure decreases.

Please log inor registerto add a comment.

Raoult's law and non volatile solutes;

Unable to watch the video, please try another server.

Unable to watch the video, please try another server.

This implies both the solute and the solvent takes the same amount of energy to escape to the vapour phase as when they are in their pure states.

Therefore, the vapor pressure of the solution will be the vapor pressure due to solvent only.

Raoult's law states that the relative lowering of vapour pressure of a solution containing a nonvolatile solute is equal to the mole fraction of the solute in the solution.
Therefore, the vapor pressure of the solution will be the vapor pressure due to solvent only.

For a nonvolatile solute bin a liquid solvent a, the relationship between concentration and vapor pressure is expressed by raoult's law:

(1) p s o l u t i o n = χ s o l v e n t p s o l v e n t o

All of the unknown substance remains in solution.

The decrease in vapor pressure is directly proportional to the mole fraction of solute in an ideal solution;

Raoult's law there are several ways of stating raoult's law, and you tend to use slightly different versions depending on the situation you are talking about.

Raoult's law is valid only in the case of ideal solutions.

Also give any two limitations of raoult's law.

Nonvolatile and volatile solutes 16 of 20 > review constants periodic table as the concentration of a solution increases its vapor pressure decreases.

Raoult's law is a thermodynamic law that states that the partial vapor pressure of each component of an ideal mixture of liquids equals the vapor pressure of the pure component multiplied by the.

Describes raoult's law and gives specific examples.

In a solution with a nonvolatile solute, only the pure vapor of the solvent is present above the solution.

The lowering of vapor pressure depends on the mole fraction of the solute dissolved and the vapor pressure of the pure solvent.

I know that raoult's law holds true only for a non volatile solute in a volatile solvent mixture wherein the vapour pressure of the solution gets lowered due to the addition of solute.

Raoults law for volatile solutes.

The lowering of vapor pressure depends on the mole fraction of the solute dissolved and the vapor pressure of the pure solvent.

Unable to watch the video, please try another server.

Raoults law for volatile solutes.
I know that raoult's law holds true only for a non volatile solute in a volatile solvent mixture wherein the vapour pressure of the solution gets lowered due to the addition of solute.

Please log inor registerto add a comment.

Raoults law for volatile solutes.
Posting Komentar untuk "Raoult's Law For Non Volatile Solute - Raoult S Law For Non Volatile Solute Youtube"